Explore the extensive list of frequently asked questions about perchlorate below, separated by issue area.
Basics
Water
Health
Regulation
Is perchlorate also naturally occurring?
Yes. Even on Mars. Closer to home, Chilean nitrate fertilizer containing naturally occurring perchlorate has been widely used in American agriculture since the early 20th century. Though quantities used today are smaller than the amounts applied earlier in the century, the use of Chilean nitrate fertilizer in California remains substantial.
What is perchlorate?
Perchlorate is a simple salt substance made up of chlorine and oxygen. It is found in nature and can be man-made.
Is it true that perchlorate was used as a medicine?
Yes. In the 1950s perchlorate was approved by the U.S. Food and Drug Administration as a safe and effective medication to treat people with overactive thyroid glands. While it has been replaced in the U.S. with newer medications (partly because enormous doses were required to have any effect and had to be given frequently as perchlorate is rapidly eliminated from the body), [1] perchlorate is still used as a medicine in other parts of the world. Becaue of its long-standing use as a medicine, we know much more today about how perchlorate works in the body.
[1] In the early 1960s there was a concern that perchlorate might have an association with aplastic anemia. Seven patients who were being treated with perchlorate developed the disease. There were several possible reasons why, ranging from misdiagnosis of hypothyroidism to environmental concerns (the cases were clustered in two specific areas). No evidence of a connection between perchlorate and aplastic anemia has been shown. What is known is that in the four decades since, perchlorate has continued to be used and no cases of aplastic anemia have arisen among any of these patients.
What are perchlorate’s other uses?
Perchlorate is widely used by the military, NASA and the commercial space industry as an ingredient in solid rocket propellant (not “rocket fuel”) and explosives. The large amounts of oxygen in perchlorate make it an optimal oxidizer to help solid rocket propellant burn. Perchlorate is also used as an oxidizing component in safety flares, fireworks, auto air bag inflators, lubricating oils and aluminum refining. Perchlorate is naturally present in some fertilizers typically used in organic farming.
Where is perchlorate found?
For several decades, perchlorate was detected only in a few places where it was manufactured or used in large quantities. In recent years, however, new techniques made it possible to detect perchlorate in water at very low levels — below 2 parts per billion (ppb). Because of these advancements, low levels of perchlorate have been detected in more places. As perchlorate was detected at these low levels, industry and government began reviewing and studying the health effects of perchlorate, resulting in several new studies that showed these low levels have no measurable effect on human health. Treatment technologies have also been developed and implemented to address perchlorate in water.
Update: Perchlorate is less present in the environment today.
How can perchlorate be removed from water?
There are currently two major technologies in use for perchlorate removal. Ion Exchange Technology uses a resin to absorb perchlorate and remove it from water, affording the opportunity for safe and appropriate disposal of perchlorate. Biological treatment is a process that uses microorganisms to break down perchlorate into other components. In this process, water can be treated in a tank or in the ground. The primary resulting component is chloride, which is part of common table salt. Other technologies are currently under development.
How can citizens find out if there is perchlorate in their own drinking water supplies?
The easiest way to obtain this information is to call their local water company. Or, if their water service is provided by a municipal utility, citizens can contact the customer service center (usually listed on their utility bill) and get information on who their water purveyor is and how to contact them. They can also visit the water supplier’s Web site and look for either the water quality report or the Consumer Confidence Report. If a district has detected perchlorate it must be listed.
Many of these reports are also available on the EPA website »
What level is safe for pregnant women? What level is safe for children? Why not be extra cautious?
To derive the recommended “Reference Dose” – the maximum amount of a substance in drinking water that the U.S. Environmental Protection Agency (U.S. EPA) considers “safe” if consumed every day for a lifetime of exposure – the National Academies of Science (NAS) Committee selected as its starting point the No Observed Effect Level (NOEL) dose of 0.007 milligrams per kilogram per day. This level is the lowest dose used in an adult clinical study by Greer et al. (2002). At this dose, there was no difference in the amount of iodide uptake inhibition by the thyroid between people exposed to perchlorate and those not exposed to perchlorate. NAS indicated that this effect, inhibition of iodide uptake by the thyroid, is the first effect to occur before any other effects of exposure can occur. However, NAS is clear that iodide uptake inhibition is not an adverse effect (See the NAS report, “Health Implications of Perchlorate Ingestion.”) Using standard default EPA conversion practices, the RfD of 0.007 milligrams per kilogram per day is equivalent to 245 ppb in drinking water. NAS divided this dose by a safety factor of 10 to account for sensitive populations – fetuses, particularly those of pregnant women who have hypothyroidism or iodide deficiency. (See Page 172 of the NAS report, “Health Implications of Perchlorate Ingestion.”) NAS resulting recommended RfD (0.0007 milligrams per kilogram of body weight per day) would be equivalent to 24.5 ppb in drinking water.
The NAS Committee acknowledges using an unconventional approach to develop a RfD from a dose that causes no effects at all. This dose is 57 times lower than the dose that the NAS Committee identified as the minimum dose before any theoretical adverse effects of perchlorate exposure could occur (14,000 ppb).
Are newborns and children more sensitive than adults to perchlorate?
The best scientific and medical research shows that newborns and children are not affected by perchlorate at the low levels found in drinking water. Three studies conducted by Dr. Elizabeth Pearce and colleagues in 2010, 2011 and 2012 added to the scientific database of research confirming this conclusion. These studies examined thousands of pregnant women in Athens (Greece), Cardiff (Wales), Turin (Italy), Cordoba (Argentina) and Los Angeles (USA) and all had the same finding: low-level perchlorate exposure is ubiquitous in the population, but it is not associated with alterations in thyroid function during pregnancy.
Do other studies explore how perchlorate might affect newborns?
Several studies have ealuated how perchlorate might affect newborns.
- Li et al. looked at the thyroid function of newborns in Las Vegas, Nevada, where low levels of perchlorate exist in the water, and in Reno, Nevada, where there is no perchlorate in the water. In comparing the results of standard tests of newborns in the two areas, scientists found no difference between the newborns in terms of thyroid function.
- Lamm and Doemland compared counties in California and Nevada, some with trace amounts of perchlorate in the water and some without, and had similar results.
- Tellez et al. found no impacts from perchlorate on pregnant women during the critical period between the late first and early second trimesters, and no effect on fetal development or thyroid levels in newborns. The study examined pregnant women and babies from three cities in Chile, where perchlorate levels range from non-detectable to 110 ppb and daily intake of dietary iodide is equivalent with the U.S.
- Kelsh et al. evaluated whether newborns had higher rates of primary congenital hypothyroidism (PCH) or elevated concentrations of thyroid-stimulating hormones in a community where perchlorate was detected in groundwater wells. The findings, according to the Journal of Occupational and Environmental Medicine, suggest that residence in a community with potential perchlorate exposure has not impacted PCH rates or newborn thyroid function.
- Other studies show there are no measurable effects on human beings at doses up to 245 ppb.
Should pregnant women or mothers of infants take any special precautions? Where can they find further information?
As with all questions of health relating to pregnancy, prospective mothers can obtain the best possible information by speaking directly with their doctor. Information may also be obtained from the American Thyroid Association.
Are the effects of perchlorate on the body permanent?
Although there are no measurable effects when perchlorate is in the body at low levels, any effects of perchlorate on the body’s ability to produce hormones are fully reversible once exposure to high levels declines or stops.
Does perchlorate stay in the body?
Perchlorate is not stored anywhere in the body.
Are all the sources of perchlorate additive in the body?
The effect of perchlorate in the body at doses above 0.007 milligrams per kilogram per day (equivalent to 245 ppb in drinking water using EPA default assumptions) will be the same, regardless of whether its source is from drinking water or food. Perchlorate is not absorbed through the skin. To calculate the total dose from all sources, however, it is not correct to add the concentrations found in water, milk and various other foods. When calculating a total dose, the amounts of water, milk and other foods that a person ingests must be considered.
Do other substances we consume have the same effects as perchlorate?
A variety of substances found in everyday foods and drinking water can affect the body in essentially the same way as perchlorate. Nitrates and thiocyanates, which occur naturally in foods like broccoli, cauliflower, meats and leafy vegetables are all considered essential to a healthy diet, and have the same thyroid effect as perchlorate.
Much of the research available on perchlorate appears to have been funded by industry. Is this information credible?
The public can be confident in the accuracy, validity and credibility of the research. Many people believe that industry — rather than taxpayers — should fund this kind of research, but with safeguards in place to guarantee the validity and credibility of research findings. With respect to the perchlorate research referred to here, several of these safeguards are in place:
- The scientific research has been conducted entirely independent of the funding organizations.
- The research findings have been peer-reviewed by independent, neutral and respected scientists to verify the research was done correctly and the results are valid.
- These studies have also been published in internationally respected scientific journals.
This critical review ensures the studies conducted are credible and can be replicated by other scientists, now and in the future.
What has been learned about perchlorate in the past few years?
A great deal is known about perchlorate, in part because of its longstanding use as a medicine. Substantial research has been conducted since the mid-1990s to improve scientific and medical understanding of perchlorate’s health effects. These studies, particularly one by the National Academy of Science (NAS), show perchlorate causes no measurable effect on human beings at levels many times higher than the minute amounts found in the environment.
How does perchlorate affect human health?
Studies show perchlorate’s direct effects on human health are limited to the thyroid gland. High levels of perchlorate can prevent the thyroid gland from absorbing iodide (which it uses to make hormones) from the bloodstream. This is called Iodine Uptake Inhibition, or “IUI.” This is not dangerous as the body automatically compensates for IUI. Further, perchlorate-induced changes to thyroid function have not been demonstrated in studies of humans exposed to perchlorate.
Is there an adverse health effect when perchlorate inhibits the thyroid gland’s ability to absorb iodide?
“Inhibition of iodide uptake (IUI),” as this effect is called, is a routine occurrence, caused by a number of factors in our diet and environment. The body naturally compensates if the thyroid can’t absorb its normal amount of iodide from the bloodstream. The thyroid itself has an enormous iodide reserve and can open additional “channels” to let more iodide in if needed. Most Americans eat a diet with more than twice the daily need for iodide.
Has perchlorate been shown to cause cancer in humans?
No. The National Academy of Sciences has confirmed it is unlikely perchlorate causes cancer. Numerous studies have shown NO evidence that perchlorate causes cancer in humans, even when consumed at levels far higher than any found in drinking water. The State of California has also stated that perchlorate does not pose a known cancer risk to the public.
What does it take to cause an adverse health effect?
In more than 60 years if study, no research has shown perchlorate causes adverse health effects. Theorectically, a sequence of three events would be required for perchlorate to have a potential adverse effect on health, each requiring higher doses.
First, perchlorate exposure must be high enough to prevent the thyroid from getting its usual amount of iodide. This may begin to happen at around 245 ppb of perchlorate in drinking water. Second, exposure must be high enough to overwhelm the body’s normal adaptive process, thereby lowering the amount of thyroid hormones in the body (scientific research indicates this does not occur at levels below 14,000 ppb). Third, exposre must be sustained long enough to reduce thyroid hormone levels for a long period of time. An adverse health effect would require daily consumption of more than 14,000 ppb in drinking water. To put this in perspective, more than 98% of perchlorate detections in U.S. water systems are below 10 ppb1– that’s 24 times lower than the recognized no effect level.
To put this all in perspective, at 10 ppb, a human would have to drink almost 740 gallons of water a day before a health risk could be possible.
1U.S. EPA unregulated contaminant monitring rule testing, 2001 (UCMR 1)
The NAS Committee says perchlorate is “unlikely” to cause cancer. What studies did the committee review in order to reach this conclusion?
The NAS Committee stated “it is unlikely that perchlorate poses a risk of cancer in humans.” (See page 145 of the NAS report, “Health Implications of Perchlorate Ingestion.”)
“There are no reports of the appearance of a new thyroid disorder, thyroid nodules or thyroid carcinoma in any patient treated with potassium perchlorate for hyperthyroidism. Iodide deficiency in the thyroid gland, a possible consequence of perchlorate administration or exposure, is not associated with an increase in thyroid cancer … In hyperthyroid patients treated with antithyroid drugs, there was no increase in thyroid cancer mortality” (See page 62 of the NAS report, “Health Implications of Perchlorate Ingestion.”).
“The committee concludes that the thyroid tumors in the (rat) offspring were most likely treatment related but that thyroid cancer in humans resulting from perchlorate exposure is unlikely because of the hormonally mediated mode of action and species differences in thyroid function.” (see page 12)
“In addition, EPA’s science policy document on the assessment of thyroid follicular-cell tumors notes that although there may be some qualitative similarities, there is evidence that “humans may not be as sensitive quantitatively to thyroid cancer development of thyroid-pituitary disruption as are rodents. The increased sensitivity may be due to marked species differences in the physiology of the thyroid gland. The EPA and IARC documents provide guidance for the evaluation of thyroid follicular-cell tumors based on mode of action (for example, tumors secondary to hormone imbalance).” (see page 145)
One of the animal studies of perchlorate looked at two generations of rats that were exposed to perchlorate in drinking water. A certain type of thyroid tumor known as a follicular cell tumor was identified in two of the rats given perchlorate. On page 145 of the report, the NAS Committee indicates that these types of tumors are not unexpected in rats when they are exposed to agents that affect the thyroid because “spontaneous thyroid follicular-cell adenomas can occasionally be observed in control rats of this strain and age.” In other words, thyroid tumors occur often in rats (especially Sprague Dawley rats as were studied here) even when they are not exposed to anything. The committee also notes that, in this regard, rats are much different than humans, stating “humans may not be as sensitive quantitatively to thyroid cancer development of thyroid-pituitary disruption as are rodents…The increased sensitivity may be due to marked species differences in the physiology of the thyroid gland.”
Rats are known to respond much more rapidly and to a greater extent to agents that affect their thyroid than do humans because of many physiological differences. When a rat is exposed to an agent that affects the thyroid, more thyroid stimulating hormone (TSH) is produced than in a human at a comparable dose, which causes a much more rapid production of thyroid cells. When cells multiply rapidly, the likelihood that cellular mutations will occur increases, which can lead to tumors. Since TSH levels are not affected in humans at equivalent doses, stimulation of the thyroid to produce more cells does not occur. The NAS states that these agents “can be assumed not to be carcinogenic in humans in concentrations that do not lead to alterations in thyroid hormone homeostasis.” (See page 145 of the NAS report, “Health Implications of Perchlorate Ingestion.”)
What is a simple definition of “point of departure?”
In risk assessment, the “point of departure” is the dose selected as the starting point to which uncertainty or safety factors are applied to derive a “safe” dose.
See the EPA’s definition of “point of departure” on their website »
Historically, this starting point is either the No Observed Adverse Effect Level (NOAEL) or the Lowest Observed Adverse Effect Level (LOAEL). The use of a No Observed Effect Level (NOEL) is a dramatic departure from current policy and constitutes a much more conservative starting point than has been used for any other regulated chemical.
What body weight should be used to calculate a safe drinking water concentration using the NAS Committee’s recommended RfD?
By definition, an RfD assumes exposure over a lifetime and takes into account special considerations of sensitive populations such as pregnant women, children, infants and fetuses. Read the U.S. EPA’s definition of a reference dose on their website » The NAS Committee calculated a RfD by starting with a No-Effect Level and then applying a 10-fold safety factor to ensure protection of potentially sensitive populations. If this value was used to calculate a safe drinking water concentration, any additional adjustment to account for sensitive populations, such as using body weights and drinking water consumption rates for the sensitive population, would be unnecessary and in essence “double counting.”
Who decides whether and how the additional studies should be conducted?
The NAS Committee made several recommendations for future research, noting that sufficient data now exists to move forward with the regulatory process. Since the NAS report, multiple studies have filled the few data gaps identified by NAS and have confirmed the NAS conclusions.
In July 2020, under the first Trump Administration, U.S. EPA made a determination under the Safe Drinking Water Act that that perchlorate did not merit additional federal regulation. That determination was subsequently upheld in 2022 under the Biden Administration.
What is the Part Per Billion (ppb) level in drinking water that equates to the NOEL as defined by the NAS Committee?
EPA uses standard default conversion factors for body weight (70 kilograms) and tap water consumption (2 liters per day) to translate RfDs to drinking water equivalents in ppb. For most people in a population, these assumptions are conservative as most people do not drink this much water each day (84 percent of the population drinks less than this amount each day). The following ppb equivalents correspond to various perchlorate doses:
PPB-EQUIVALENTS FOR VARIOUS DOSES
| No Observed Adverse Effect Level (NOAEL) [0.4 mg/kg per day] | 14,000 ppb |
| No Observed Effect Level (NOEL) [0.007 mg/kg per day] | 245 ppb |
| NAS Committee recommended reference dose [0.0007 mg/kg per day] | 24.5 ppb |
The NAS Committee based its RfD on the NOEL instead of the NOAEL and applied an additional 10-fold safety factor to account for sensitive populations.
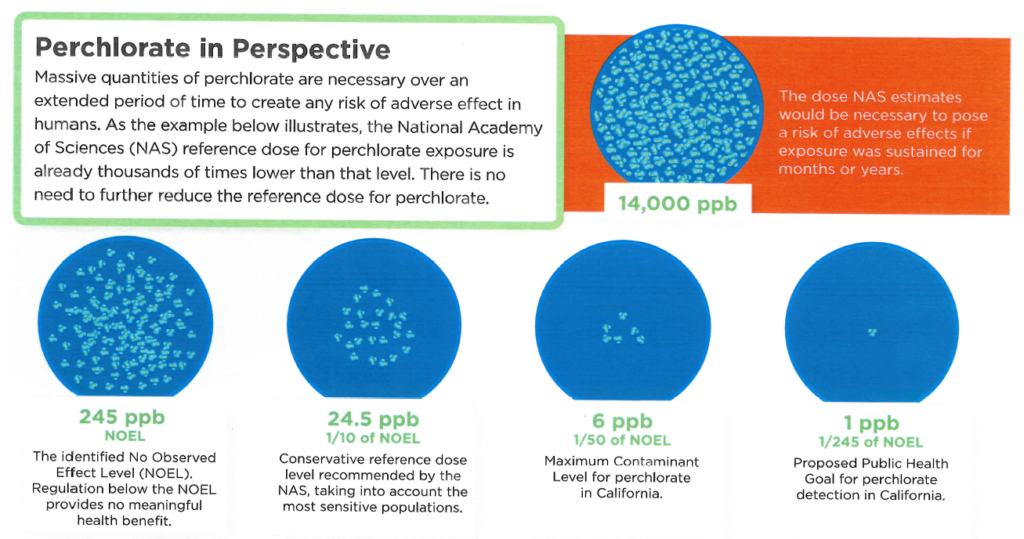
What is California’s “safe” exposure level and how was it derived?
In 2007, the State of California —accounting for perchlorate exposure from water, farm products and cow’s milk — enacted a Maximum Contaminant Level (MCL) for perchlorate in drinking water of 6 ppb. This is an exceptionally conservative approach considering studies show perchlorate begins to affect the thyroid at approximately 245 ppb, and below this level there is no measurable effect. No studies show environmental levels of perchlorate cause harm to human health.
Are the federal 24.5 ppb Drinking Water Equivalent Level (DWEL) and California’s MCL “final” standards?
California’s current MCL is “final” in that it is currently enforceable, but is under review by regulatory authorities as part of a review process required by state law. At the federal level, the U.S. EPA has decided to set a national drinking water standard for perchlorate, but has not yet indicated what standard will be proposed.
What are the next steps in California’s regulatory process for perchlorate?
Following a mandatory five-year review mandated by state law, California proposed revising its Public Health Goal (PHG) for perchlorate from 6 ppb to 1 ppb. The State Water Resources Board is now conducting research to determine how frequently perchlorate is found in drinking water and whether perchlorate occurs frequently enough to merit further ation.
What are the next steps in U.S. EPA’s regulatory process for perchlorate?
EPA’s 24.5 ppb reference dose (the maximum dose without any risk, abbreviated RfD) may be used by officials across the U.S. to make site-specific cleanup or interim drinking water standard decisions involving perchlorate. States and private parties also may look to EPA’s RfD and Drinking Water Equivalent Level (DWEL) to make similar decisions.
In July 2020, under the first Trump Administration, U.S. EPA made a determination under the Safe Drinking Water Act that that perchlorate did not merit additional federal regulation. That determination was subsequently upheld in 2022 under the Biden Administration .
However in 2023, litigation that focused on EPA’s administrative requirement to set a standard (rather than the actual science or health implications of perchlorate itself resulted in a ruling that compels EPA to issue a “proposed” National Primary Drinking Water Regulation for perchlorate by November 21, 2025, and a final regulation by May 21, 2027.
This begins a public process with opportunities for stakeholder input, EPA may request extensions to these deadlines, however as of June 2025 the agency has reported it is on track to meet them.
What are the impacts of unnecessarily restrictive standards?
The potentail costs of drinking water standards more restrictive than what credible science says is necessary to protect public health are staggering. California, Nevada and Arizona could be the states most impacted. Misguided standards would in effect create a “problem” where one does not really exist, forcing citizens, industry and government to incur significant expenses for new treatment plants, retrofitting existing treatment plants, purchasing additional water supplies, lowering reservoir levels and pumping more groundwater from existing sources. This substantial expense of resources would have no public health benefit.
To implement perchlorate compliance strategies would mean substantial costs for small water systems, with conceptual costs above $3 per 1,000 gallons for very small systems (serving less than 500 people). While the total costs for the MCL are low, the cost burden would be primarily placed on a small number of water systems and their customers.
What is being done now to address perchlorate in drinking water supplies?
Perchlorate is being contained, removed and treated at sites where it has been used for manufacturing. New state-of-the-art technologies for removing perchlorate from water have been developed and put to use. Others are being developed or refined.
The EPA and the NAS Committee recommended a reference dose. What is a reference dose?
RfD is defined by the EPA as, “an estimate of a daily oral exposure to the human population (including sensitive subgroups such as children) that is not likely to cause harmful effects during a lifetime.”
See the definition of reference dose on the EPA website »
EPA’s definition is based on the assumption that exposure could occur throughout a lifetime and takes into account all stages of life. The definition also takes into account “sensitive subgroups,” such as pregnant women, infants, children and fetuses.
It is sometimes misunderstood to mean that doses above the RfD are unsafe. This is not correct. The RfD incorporates a number of safety factors to ensure the value is health-protective. When a dose exceeds an RfD value, it does not mean that an adverse effect will occur. Learn more about safe water from the EPA »
The RfD is not a regulatory standard —- it is one of the building blocks assembled into a regulatory standard, and should not be misinterpreted as serving the purposes of a regulatory standard.
How does EPA derive an RfD, and how did the NAS Committee derive one in the case of perchlorate?
Typically, EPA derives an RfD by starting with the highest dose at which no adverse effects can be observed (the No Observed Adverse Effect Level, or NOAEL). Learn more abou how the U.S. EPA sets RfD»
It is unprecedented to use the level at which no effect, even a mundane, biochemical effect, is observed (the No Observed Effect Level, or NOEL); however, this is the starting point that the NAS Committee used for perchlorate. The NAS Committee defined a NOEL as “the highest dose at which there are no statistically or biologically significant increases in the frequency or severity of any effect between the exposed populations and its appropriate control.” (See page 168 of the report, “Health Implications of Perchlorate Ingestion.”)
Where a NOAEL is used, uncertainty factors are customarily applied to the starting dose to account for scientific uncertainty and ensure the RfD protects public health. For example, to account for sensitive populations such as pregnant women, children, infants and fetuses, the EPA typically divides the NOAEL by an uncertainty factor of 3 or 10.
The NOEL dose that the NAS Committee selected as its starting point was 0.007 milligrams per kilogram per day and then divided this dose by a safety factor of 10 to account for sensitive populations. There is no precedent for this level of conservatism. The NAS Committee identified the NOAEL for perchlorate as 0.4 milligrams per kilogram body weight per day, stating that, in adults, “sustained exposure” (i.e., several months or longer) to more than 0.4 milligrams per kilogram of perchlorate per day is likely required before any harm could occur.
Alternatively, if an uncertainty factor of 3 or 10 for sensitive populations was applied to the NOAEL the reference dose could have set at 1,400 to 4,655 ppb in drinking water.
What is a NOEL?
The NAS Committee defines a NOEL as “the highest dose at which there are no statistically or biologically significant increases in the frequency or severity of any effect between the exposed populations and its appropriate control.” on page 168 of the report, “Health Implications of Perchlorate Ingestion.” The NAS committee states that a dose of 0.007 milligrams per kilogram of body weight per day is the NOEL. Using standard default EPA conversion practices, this is equivalent to 245 ppb in drinking water.
What are “uncertainty” or “safety” factors?
As with most issues in life, health and safety, decisions must be made absent absolute certainty. “Uncertainty” or “safety” factors account for a lack of scientific data or to address possible variability in population sensitivity when deriving a safe dose level.
Is it appropriate to apply a safety factor to a NOEL? Is there a precedent for this? Are there scientific arguments against it?
Applying a safety factor to a NOEL is unusual and no precedent has been identified.
The NAS Committee acknowledges its recommendation “differs from the traditional approach.” (See page 15) Moreover, the NAS Committee does not provide supporting precedent to justify applying a safety factor to this kind of dose. In this instance, the NAS Committee recommends a reference dose more than 570 times lower than the dose it concludes does not cause adverse effects in humans.
